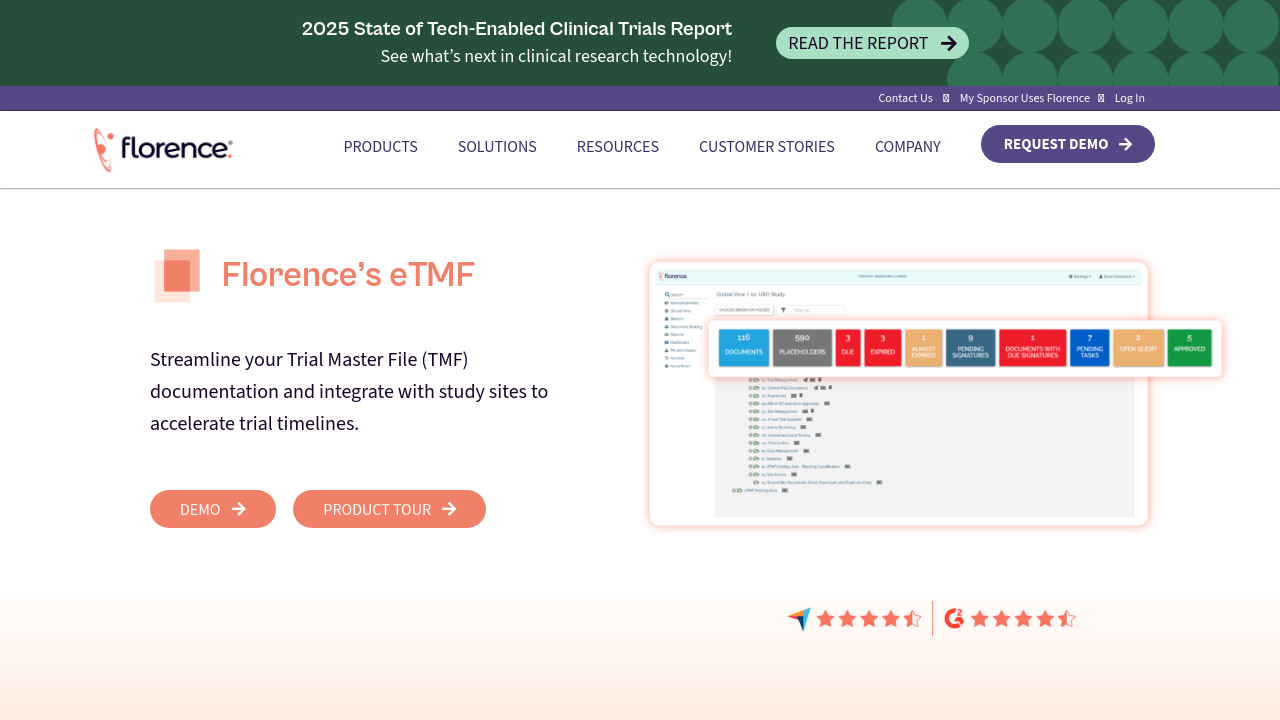
Florence eTMF is a cutting-edge Electronic Trial Master File solution designed to streamline the way research teams manage clinical and regulatory documents. Developed by Florence Healthcare in Atlanta, Georgia, this innovative system bridges the gap between home health care data and the broader health care community, ensuring that clinical trials run efficiently and remain compliant.
Florence eTMF stands out for its unique ability to combine ease of use with integrated remote access to study sites. With over 10,000 research sites connected to the Florence Network, the software offers a centralized platform for Sponsors and Contract Research Organizations to manage, share, and retrieve essential documents seamlessly. This centralized document management system supports quick document uploads and retrievals, simplifies audit trails, and enhances collaboration among study sponsors, clinical sites, and regulatory stakeholders.
Key Features and Benefits:
- Integrated Remote Access: Enable instant connectivity with study sites for seamless monitoring of essential documents and trial records.
- User-Friendly Interface: Designed for intuitive navigation, Florence eTMF facilitates easy document uploading, editing, and digital signature collection while maintaining strict regulatory compliance.
- Centralized Document Management: Consolidate all trial documents into a secure, single location, improving oversight and audit readiness.
- Efficient Workflow: Streamline document submission and review processes with features designed to minimize administrative delays and reduce error-prone manual entries.
- Regulatory Compliance: Ensure 21 CFR Part 11 compliance with secure, tamper-evident electronic records, protecting the integrity of clinical data.
Florence eTMF not only enhances internal processes for clinical trial management but also provides significant benefits in remote connectivity and data integrity. Its flexibility allows customization to meet specific study protocols, and ongoing enhancements ensure the platform evolves with the latest industry requirements. Research teams can quickly move from paper-based systems to a modern, digital environment, ultimately reducing document management timelines and expediting trial start-up periods.
Florence eTMF belongs to the Research Laboratory Information Systems category. It’s an ideal solution for research professionals who demand a powerful yet simple-to-use system to manage trial master files. Whether you are a clinical trial project manager, a regulatory affairs professional, or part of a monitoring team, Florence eTMF provides a robust platform that adapts to your workflow.
Explore exclusive deals by visiting the Florence eTMF Deal Page and learn how this innovative solution can transform your clinical document management.
Top Alternatives to Consider: